Sorry, that story no longer exists on our website
The article or video you are looking for has been moved to our archive. Perhaps another story on this page might interest you?
Scroll down for links to our latest news, crisis explainers and videos from our projects.
Latest news and stories from MSF UK
MSF calls for rapid scale-up of humanitarian response amid inaction from the UN and international organisations
Learn more

Dr Priscille Cupidon reports from Port-au-Prince where MSF teams are working to reach and treat people cut off from care
Learn more

MSF staff and patients faced weeks of siege, trapped inside what was the largest hospital in south Gaza
Learn more
Never miss another story
Sign up to Frontline, our monthly e-newsletter, to stay up to date with MSF's life-saving work in over 65 countries
MSF crisis responses explained
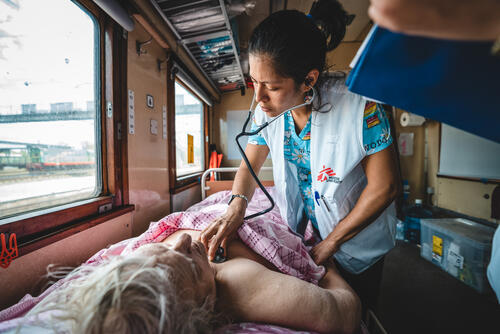
MSF teams are working to deliver medical aid to people in Ukraine and neighbouring countries
Learn more

COVID-19 has made the collapse of Yemen's health system complete, following five years of conflict
Learn more